Elevating Compliance and GxP in regulated industries
ComplianceWire helps scale trainings to meet regulatory and industry standards in a FDA CFR Part 11 validated system. Navigate regulations, automate training and implement risk management to support development of safer and more effective medical products.
Gain oversight into your employee qualifications and training
Track and monitor the status of your employees in meeting their required qualifications and training in aggregate, or drill into the areas where improvement possibilities and risks are identified.
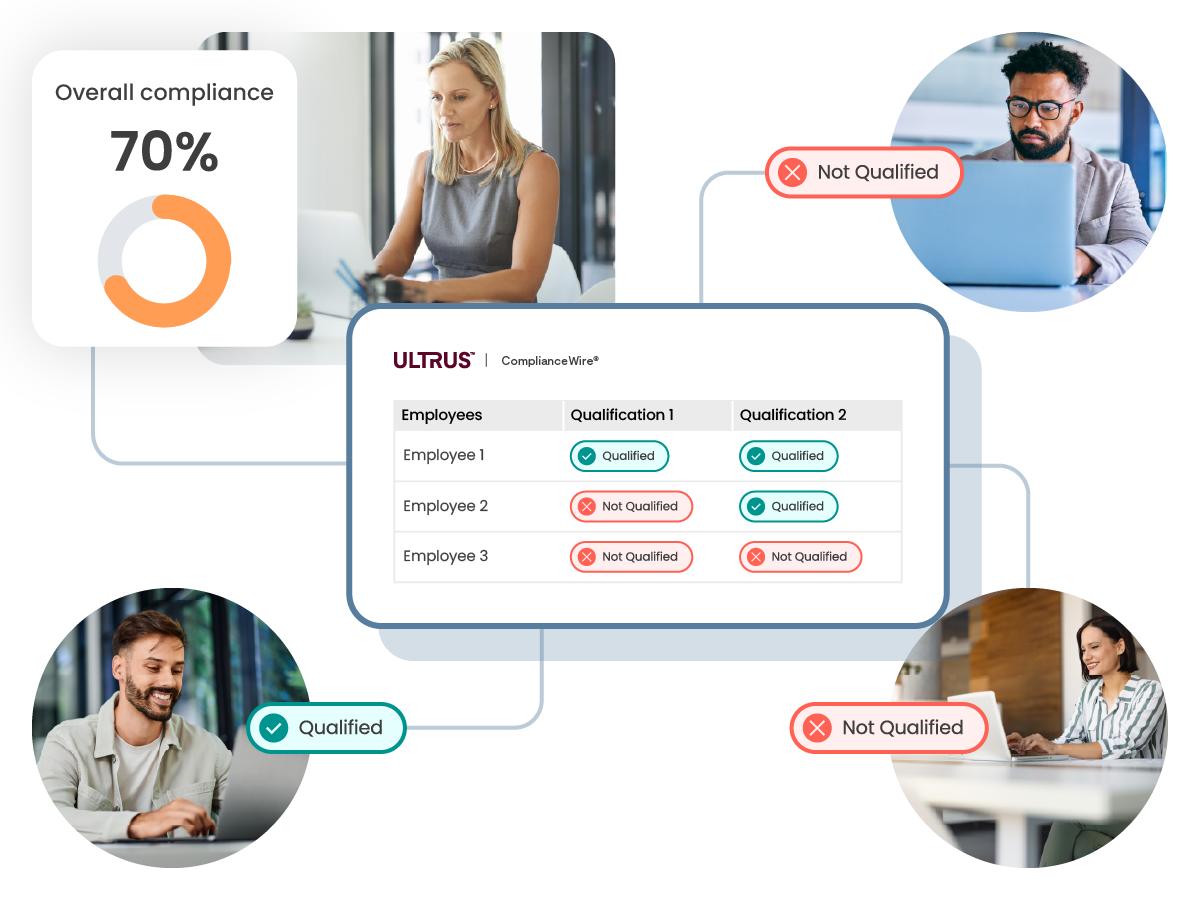
Qualification overview
Dashboards and reports provide an organization-wide view of compliance and qualification status.
Qualification matrix
Drill down into each team and qualification in real time to understand exactly what is missing that is preventing compliance.
Schedule reports
Schedule reports to team leaders to support the active promotion of training and qualifications.
Streamline and automate your employee qualification process
Easily communicate with and monitor your employees and follow up on post-audit findings (CAPAs) and other regulatory obligations. Upload required training, qualifications and policies to maintain compliance.
Create and augment
Easily create your own courses or edit and re-brand our off-the-shelf library to cater the content to your organization.
Automate training
Users can easily engage in dynamic eLearning courses to scale up training.
Fully configurable
Create different mechanisms for standards to meet compliance, build out team hierarchies and integrate with your wider ecosystem.
Be ready to respond to an audit or regulatory inquiry
ComplianceWire is natively compliant with FDA 21 CFR Part 11 standards and contains many of the requested reports from regulatory agencies right out of the box. Respond to authorities and internal audits confidently.
Validated system
Learn how UL Solutions verifies every major release of ComplianceWire®, with insights from our Strategic Advisory experts.
Audit readiness support
Leverage the ComplianceWire team to prepare for and support regulatory inquiries.
Proven trust
ComplianceWire is regularly audited internally and by our customers. On average, our customers audit ComplianceWire over 50 times a year, including paper, remote and onsite audits.
Grow expertise with ComplianceWire’s e-Learning Library for Life Sciences
Get help to remain compliant with the U.S. Food and Drug Administration (FDA) regulation of medical devices and pharmaceuticals. Our course catalog contains more than 400 life sciences e-learning courses, many of which were co-developed with the FDA.
Extensive coverage
UL Solutions offers over 1,000 standard regulatory and knowledge-focused e-learning courses, including 21 CFR part 820 training, 508 compliance e-learning, CAPA training and more.
Regulatory expertise
UL Solutions expertise combined with FDA-authored and/or reviewed courses, identical to those used by the FDA to train its inspectors and investigators.
Engaging content
Includes stylized topic-specific videos, embedded interactions, and quizzes, all implemented by skilled instructional designers.
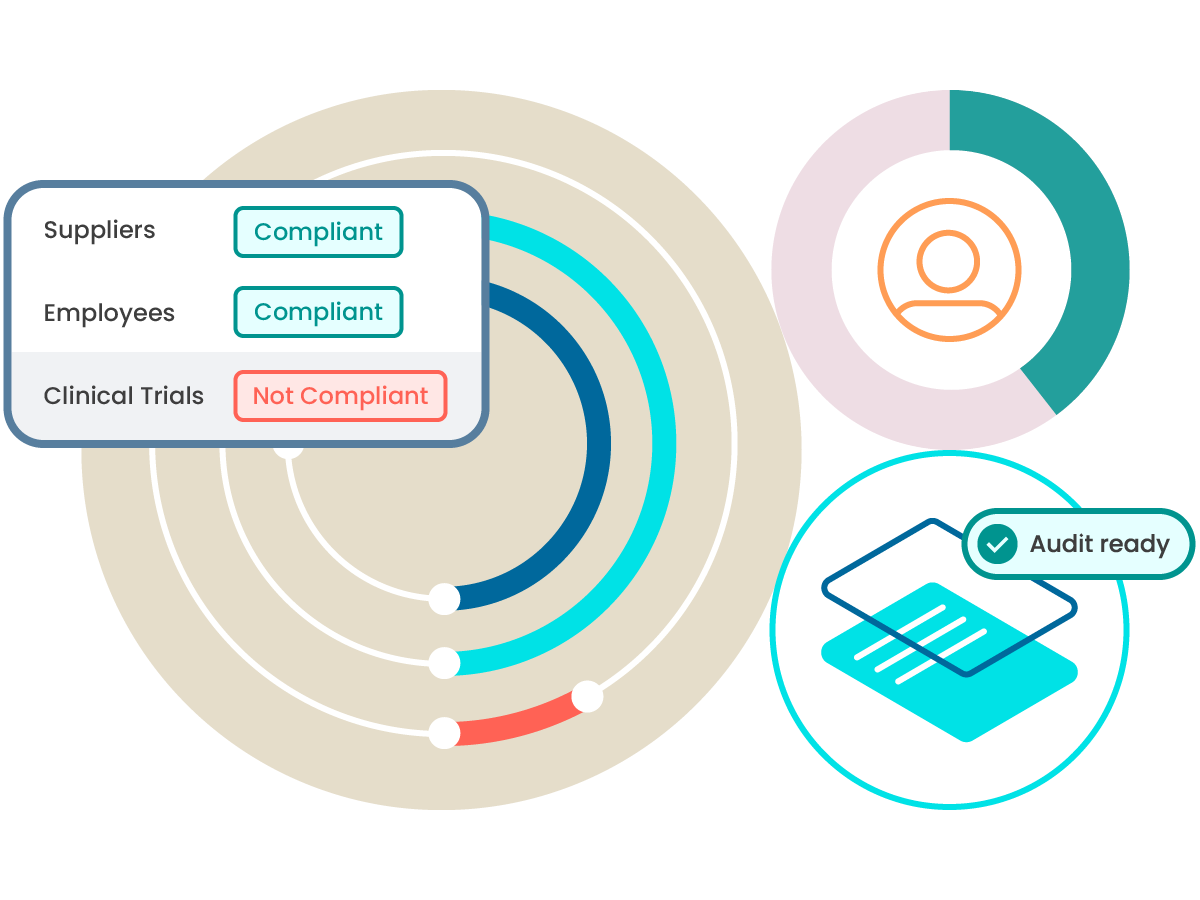
Scale quality and compliance across your business processes
Elevate quality from being a manufacturing-only focus to being a central pillar that helps reduce risk across the entire business.
Supplier qualification
Learn how ComplianceWire can help support qualification and quality across your supply chain.
Clinical trials
Support positive patient outcomes and help clinical research by covering qualifications across clinical trials.
Social responsibility
Help support employees, suppliers, partners and vendors who are meeting the standards of excellence associated with your brand.
ComplianceWire® and ULTRUS™ software
ComplianceWire® is now part of ULTRUS software, which brings together flagship digital offerings from UL Solutions to help customers manage their regulatory, supply chain and sustainability challenges.
Ready to boost your life sciences training and e-learning capabilities?
Schedule a demonstration to learn how ComplianceWire can help you navigate complex regulatory requirements.